Seeking peace of mind, some Minnesotans skirt COVID-19 booster guidelines

Go Deeper.
Create an account or log in to save stories.
Like this?
Thanks for liking this story! We have added it to a list of your favorite stories.
In August, Gary Spielman, who has a history of cancer, went to a CVS near his home in Coon Rapids for his third dose of the Moderna vaccine. It came on the recommendation of his doctor.
Gary's wife, Rose, went with him. With her immune system intact, Rose wasn't eligible to get a shot that day. But as the couple was waiting the required 15 minutes after Gary’s shot, Rose asked the pharmacist if she could get a Moderna booster.
“She said ‘oh sure,’” Rose said. “She gave me some paperwork and it was all about your immune system and I checked ‘no’ to every single thing.”
“There were no questions asked,” Gary said. “We don’t know why. Maybe they had extra vaccines.”
Turn Up Your Support
MPR News helps you turn down the noise and build shared understanding. Turn up your support for this public resource and keep trusted journalism accessible to all.
Frustrated with the pace of the federal government's approval for vaccine boosters, seeking peace of mind or just a sense of normalcy, an unknown number of Minnesotans have stepped ahead of federal guidance on COVID-19 boosters, instead taking their health decisions into their own hands by finding second or third shots at willing pharmacies or other vaccine providers.
Right now a limited number of people are eligible for additional doses of the Moderna or Pfizer vaccine if, like Gary Spielman, they have a compromised immune system.
And last week, the Centers for Disease Control and Prevention gave the green light for older or high-risk people to get a Pfizer booster.
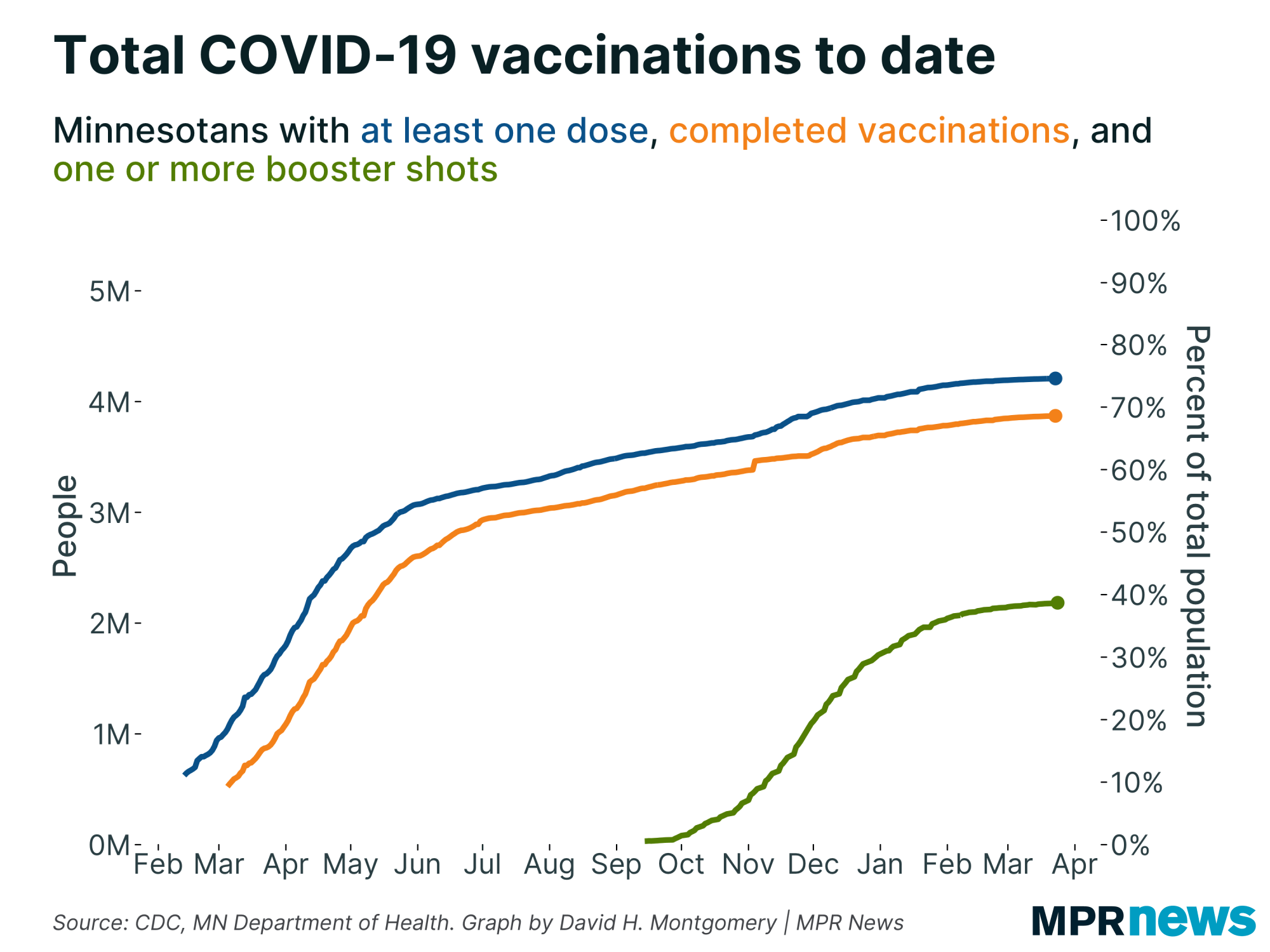
About 44,000 immunocompromised people in Minnesota have gotten third doses. Since the CDC authorized Pfizer boosters, an average of about 4,400 are being given daily, according to the Minnesota Department of Health.
For Rose Spielman, who is 68, she says getting that extra Moderna shot was a matter of protecting her husband. She didn't want to be the source of inadvertent transmission of the virus.
“I just don't want to risk anything, me getting really sick with him being so compromised,” she said.
But it was also a practical matter of wanting to feel safe while inching back into her pre-pandemic life.
"Other family members that I know have not had any vaccines at all on my side and it's really frustrating for me to talk to them or get together with them without them being immunized at all."
Effectiveness fears
Annie Boehm, 62, said she’s a big believer in everyone being vaccinated to keep the community at large safe. So last spring, Boehm, of Northfield, said she was happy to get the single-dose shot of the Johnson & Johnson vaccine.
“I wholeheartedly endorse people getting vaccinated even if they are not worried about getting sick,” she said.
But as the summer wore on, and cases rose again driven by the highly contagious delta variant, Boehm worried that her shot might not work well enough.
“I’m really most concerned about my health and my husband's,” she said.
With news that there were thousands of unused vaccines on the shelves, Boehm went to a CVS in her local Target to start the two-dose course of the Moderna vaccine, which she’d read was more effective than the Johnson & Johnson shot.
Boehm said the pharmacist didn’t ask questions about her vaccine history.
“They didn't ask and I didn't tell,” she said.
Research shows that all three vaccines — Johnson & Johnson, Pfizer and Moderna — are highly protective against severe illness, hospitalization and death from the virus, and that boosters may only be necessary for a subset of the population — a matter of heated debate among experts advising federal officials on their vaccine protocols.
But research also shows that months later all three vaccines are somewhat less effective than they were when they were first given to people, with some doctors specifically recommending boosters for those who got Johnson & Johnson shots soon.
In Minnesota, less than 1 percent of vaccinated people have contracted COVID after vaccination, and a tiny fraction of them have been hospitalized or have died.
Boehm reviewed this research, including news that other countries are advising recipients of single-dose vaccines similar to the Johnson & Johnson shot to follow up with a Moderna or Pfizer vaccine, and reasoned that getting another round would only help her and those around her.
"I'm no scientist, but I'm a big believer in science. And the fact that the science is clear enough was enough for me,” she said.
What the data says
Minnesota Department of Health infectious disease director Kris Ehresmann warned against veering outside federal booster shot guidelines.
"We don't have the science to suggest that that will be beneficial to them, and we don't have data to suggest that that is safe for them,” she said.
Ehresmann said it's important that people who need boosters are getting them at the right time. And it's unclear whether it's safe to mix vaccine brands.
Further, Ehresmann says that pharmacists giving extra shots to people who aren’t eligible to get them risk losing their licenses because they've signed a contract with the state to administer vaccines appropriately.
She added that going outside federal guidance risks eroding the integrity of the scientific process behind the vaccines.
“We've got people who say, ‘Oh, I don't trust the data, and I'm not going to get it.’ And then we've got some people say, ‘You're not giving me enough of these vaccines, and you're not giving it to me fast enough,’” she said. “We go back to the same message: We're looking at the science, and we're looking at the data and our recommendations are based on that."
Soon, Ehresmann said, federal officials will review boosters for the Moderna and Johnson and Johnson shots, too.


